
When an ion keeps the same charge in a reaction and is just there to balance out the opposite charges, we call it a Spectator Ion. Nitrate ion in both the reactants and the products has a -1 charge. In the equation below, nitrate is there to balance the charges.

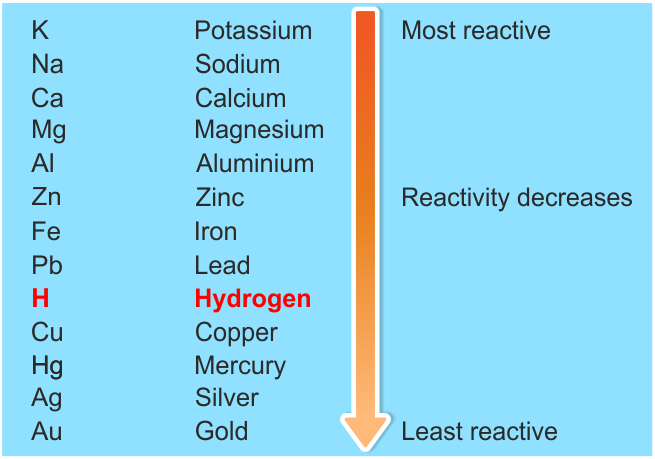
Positive ions (cations) can't just be floating around by themselves in solution, they have to have a negative ion (anion) to balance them. We say that Zn is more active than the copper. “ Reactivity metals in dilute sulfuric acid” By Capaccio – Own work (CC BY-SA 3.\): An activity series for common metals.Įxample: Zn and Cu both tend to lose 2 electrons, but the zinc wants to lose them more, so much so that it can force the two electrons on Cu 2 + and make it become Cu again. “ Reactivity Series – Reactivity of Metals Chart, Features, Uses.” BYJUS, 18 Aug. The key difference between electrochemical series and reactivity series is that electrochemical series gives the order of the standard electrode potentials, whereas reactivity series gives the arrangement of metals in the descending order of the reactivity of those metals. Reactivity series of metals is also known as the activity series and describes the arrangement of metals in the descending order of the reactivity of those metals. Summary – Electrochemical Series vs Reactivity SeriesĮlectrochemical series is a list of chemical elements that shows the order of standard electrode potentials of them. The below infographic presents the differences between electrochemical series and reactivity series in tabular form for side by side comparison. What is the Difference Between Electrochemical Series and Reactivity Series?Įlectrochemical series and reactivity series are important listings of chemical elements electrochemical series includes chemical elements with electrode potentials, while reactivity series includes metals. In this series, hydrogen is included, although it is not a metal because it is used as the standard for comparison. Also, we can use this reactivity series to obtain information on the reactivity of metals towards water and acids.įor example, metals like potassium, sodium, lithium, and strontium react with water, and metals like magnesium, aluminum, manganese, zinc react with acids, whereas metals such as antimony, bismuth, mercury, and silver are highly unreactive. In other words, we can determine whether a metal can displace another metal in a single displacement reaction. We can use the details given by the activity series to predict the displacement ability of a metal. Reactivity series of metals is also known as the activity series, and it describes the arrangement of metals in the descending order of the reactivity of those metals.
We can measure this value by taking the particular metal as the cathode and the standard hydrogen electrode as the anode. Moreover, this series gives the electrode potential of these chemical elements, and the list is arranged according to the standard electrode potentials. Because of their low reactivity, we often use them to make coins, jewelry, etc., so we call them “noble metals.” Examples include copper, gold, silver, etc. They are relatively very stable and do not form compounds readily. These metals are thus called “active metals.”Īt the bottom of the series, there are transition metals. Moreover, they easily react to form compounds. These are more reactive and easily undergo oxidation than metals at the bottom. Another common name for this series is “ activity series.” Furthermore, this series lists metals in order of decreasing reactivity.Īt the top of the series, it has alkali metals and alkaline earth metals. It provides enough information about the relative reactivity of metals in aqueous solutions under standard conditions. Summary – Electrochemical Series vs Reactivity Series What is Electrochemical Series?Įlectrochemical series is a list of chemical elements that shows the order of their standard electrode potentials. Electrochemical Series vs Reactivity Series in Tabular Formĥ. The key difference between electrochemical series and reactivity series is that electrochemical series gives the order of the standard electrode potentials, whereas reactivity series gives the arrangement of metals in the descending order of the reactivity of those metals.Įlectrochemical series and reactivity series are important listings of chemical elements electrochemical series includes chemical elements with electrode potentials, while reactivity series includes metals.


 0 kommentar(er)
0 kommentar(er)
